Abstract
Background There is a shift in treatment paradigm for elderly transplant ineligible (TI) population in the recent years. Historically lenalidomide plus dexamethasone (Rd) served as reference treatment for patients with newly diagnosed multiple myeloma (NDMM). Lenalidomide, bortezomib, dexamethasone (RVD) has been proposed due to its favourable risk-benefit profile, with data supporting its use in younger patients without intent for frontline transplant. Bortezomib in combination with cyclophosphamide and dexamethasone (VCD), commonly used in Australia and New Zealand, is an acceptable alternative demonstrating reasonable real-world efficacy not dissimilar to established regimens. However, there has not been any head to head comparison between RVD and Rd, or between either of these with VCD in elderly TI NDMM patients. We aim to evaluate the efficacy of these 3 regimens using data from the Myeloma related diseases registry (MRDR). The difference in funding, where New Zealand patients receive predominantly frontline VCD, while Australians are given Rd and more recently RVD, has created a unique opportunity to evaluate a large cohort of patients in the real-world setting.
Method All patients with a confirmed diagnosis of multiple myeloma from Australia and New Zealand aged above 70 in the MRDR were identified. Cases were included in this analysis if they received VCD, Rd or RVD as first line treatment. Any patients who received autologous stem cell transplant either in first or second line of treatment were excluded. Patient characteristics across these 3 treatment cohorts were compared using Mann-Whitney U test and Chi Square test where appropriate. Progression-free survival (PFS) was calculated from time of diagnosis to either progression or death. Patients who had change of treatment prior to progression were censored when second line treatment was started. Overall survival (OS) was measured from diagnosis to death. Survival analysis was performed Kaplan-meier analysis and log-rank test were used for comparison. Cox-regression analysis was used for multivariant analysis. All statistical analysis was done using IBM SPSS version 27, and a p-value of <0.05 was deemed statistically significant.
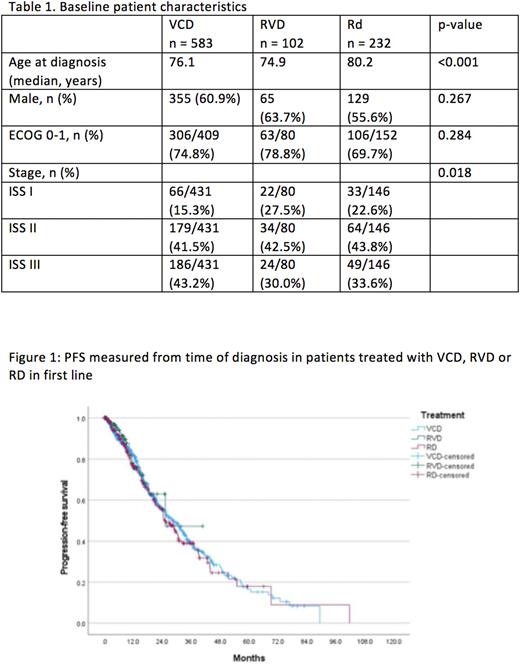
Results Between March 2011 and March 2022, a total of 3602 patients with a confirmed diagnosis of multiple myeloma from Australia and New Zealand were enrolled in MRDR. Amongst these patients, 1516 (42.1%) were aged above 70, and 1011(66.7%) received either VCD (583), Rd (232) or RVD (102) as first line treatment. Ninety-two patients subsequently received ASCT and were excluded, leaving a total of 919 patients for this analysis. The median age of the entire cohort was 76.8 years, and 59.9% were males. Amongst those where data was available 18.4% were ISS-I, 42.2% were ISS-II, and 34.9% had a ECOG of 2-4. Patients who received Rd were older, and there was a higher proportion of patients with ISS stage III disease in the VCD group (table 1). The median follow-up using inverse censoring rule was 28.7 months for the entire cohort with the RVD group having the shortest median follow-up at 11.3 months. The median PFS was similar across all treatment groups (VCD 28.3 mth, Rd 25.3mth, and RVD 25.3mth, p = 0.833, figure 1), and no difference in OS was noted (VCD 52.1mth, Rd 42.8mth, and RVD NR, p = 0.411). Even after adjusting for age, ECOG and ISS, no difference between Rd and the other two treatment groups were noted for PFS (VCD HR 1.11, 95% CI 0.87 - 1.42; RVD HR 1.24, 95% CI 0.74 - 2.08) and OS (VCD HR 1.00, 95% CI 0.75 - 1.33; RVD HR 1.22, 95% CI 0.61 - 2.44).
Conclusions Based on real world Australasian data, our study highlights that there is no difference in outcomes between RVD, Rd and VCD in elderly transplant ineligible population with myeloma. However, this observation may be limited by shorter follow up time for RVD patients. A longer follow up may provide useful data and guide future clinical practice.
Disclosures
McQuilten:Sanofi: Research Funding; Novartis: Research Funding; Janssen: Research Funding; GSK: Research Funding; BMS/Celgene: Research Funding; CSL: Research Funding; Beigene: Research Funding; Abbvie: Research Funding; Amgen: Research Funding; Takeda: Research Funding. Wood:Amgen: Research Funding; Abbvie: Research Funding; Sanofi: Research Funding; Novartis: Research Funding; Roche: Research Funding; Gilead: Research Funding; Janssen-Cilag: Research Funding; CSL Behring: Research Funding; Bristol-Myers Squibb: Research Funding; Antengene: Research Funding; Astra Zeneca: Research Funding; Beigene: Research Funding. Spencer:Haemalogix: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria. Chan:Roche: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Eusa: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal